1.2: Elements of Life
Now that we understand the most important molecule for life, water, we can go over the most important atoms for life. Topic 1.2 is a crucial lesson to cover, and while it is one of the easiest topics to learn in unit 1, it provides the foundation which the rest of the unit builds upon.
Vocab List
- Essential Elements
- Macromolecules
- Organic Compound
- Hydrocarbon Chain
- Valence
- Functional Groups
- Hydroxyl Group
- Methyl Group
- Carbonyl Group
- Aldehydes
- Ketones
- Carboxyl Group
- Amino Group
- Phosphate Group
- Sulfhydryl Group
Written Explanation
There are 5 major elements of life which make up a large portion of all living things. These include carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P). Our bodies are made almost entirely out of the first 4 elements. Due to their necessity to life, these elements are called essential elements. All five elements previously mentioned make up the structures of the 4 major macromolecules, or large molecules necessary to life. This is why often you'll see the essential elements written out as CHONP (each letter standing for an element), but know that sulfur is also included (in proteins).
ProteinsStructural components of your body, enzymes, motion 
|
CarbohydratesComplex and simple sugars 
|
Nucleic AcidsStores genetic material 
|
LipidsNot traditional polymers, hydrophobic 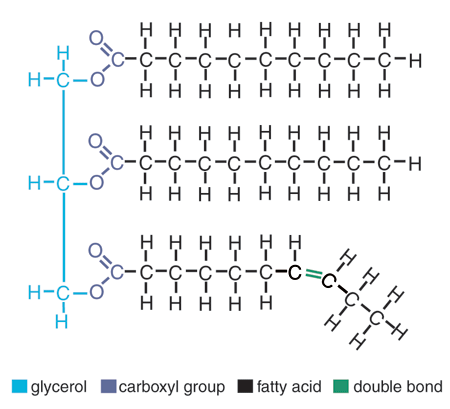
|
Properties of Carbon:
Any compound which contains carbon is known as an organic compound, otherwise known as a “living compound”. Organic compounds (but also other compounds) also always contain hydrogen. Any compound simply made out of hydrogen atoms attached to a chain of carbon atoms (bonded covalently) is known as a hydrocarbon chain. Carbon plays a special role in the buildup of macromolecules due to its ability to bind to 4 other molecules/compounds. Carbon is in group 14 on the periodic table, in which every single element has a valence of 4. This means that carbon has 4 electrons in its outermost electron shell, which can hold 8 in total. Carbon's ability to form 4 covalent bonds with other elements which can “donate” their electrons to fill carbon's valence shell allows it to form large structures which can be ringed, chain shaped, or branched. However, carbon does not need to bond with 4 other elements to fill its' shell. To attain a stronger covalent bond, an element can share 2 or 3 electrons to form double or triple bonds respectively, making carbon an even more versatile connector. This is why carbon chains or compounds act as the backbones of macromolecules.
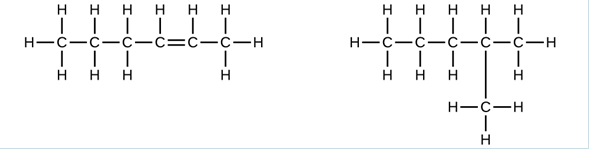 Examples of hydrocarbon chains
Examples of hydrocarbon chains
Functional Groups:
It is important to understand that the structure of the carbon backbone alone does not determine the properties of the molecule. The functional groups, which each have their own properties, also determine the properties of the molecule. These groups can determine whether the compound is basic/acidic and polar/nonpolar. The remainder of the molecule (besides the group) will often be notated as R.
The rule of thumb is that if the group contains an electronegative element such as oxygen or sulfur, the group will be polar. If it can donate or accept protons, then it will also be a charged group along with basic/acidic. If the group is just filled with hydrogen and carbon atoms then it can be considered non-polar. While these functional groups aren't necessary to memorize, it is helpful to understand the properties of each of these groups when looking at larger compounds and macromolecules further in this unit and course as a whole.