1.3: Introduction to Biological Macromolecules
All important biological molecules in your body are macromolecules. These include carbohydrates, proteins, nucleic acids, and lipids. Topic 1.3 is about the parts that make up these macromolecules, and the processes by which they are joined together.
Vocab List
- Macromolecules
- Monomers
- Polymers
- Carbohydrates
- Monosaccharides
- Polysaccharides
- Glysodic linkages
- Amino acids
- Peptide bonds
- Nucleic acids
- DNA
- RNA
- Nucleotides
- Lipids
- Dehydration synthesis
- Hydrolysis
Written Explanation
The four major macromolecules of life are carbohydrates, proteins, nucleic acids, and lipids. These molecules compose most of the biological matter in living organisms (ignoring water and inorganic matter). Most of these macromolecules are composed of smaller segments, called monomers. When joined together, the monomers become a polymer.
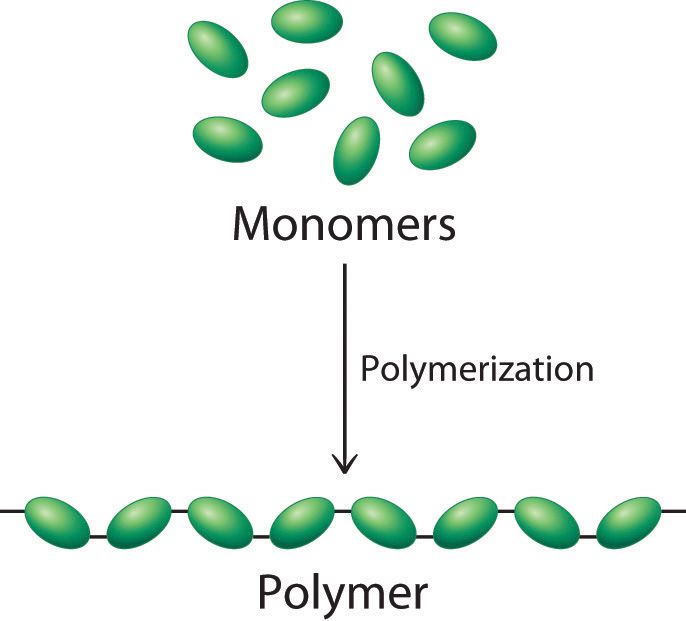 A string of monomers joined together to form a polymer
A string of monomers joined together to form a polymer
Carbohydrates (made only of Carbon, Hydrogen, and Oxygen):
The monomer unit of a carbohydrate is called a monosaccharide. The polymer is known as a polysaccharide (which is sometimes interchangeable with “carbohydrate”). Monosaccharides are either carbon chains or carbon rings.
 Two glucose monomers. A carbon chain on the left and a carbon ring on the right
Two glucose monomers. A carbon chain on the left and a carbon ring on the right
Polysaccharides are formed when monosaccharides are joined together by covalent bonds called glycosidic linkages. Polysaccharides can be very long chains or just a few monomers, but all are carbohydrates.
 A polysaccharide formed up of two monosaccharides (in this case glucose). The polysaccharide in this case is a maltose molecule and is a disaccharide (di for 2 monomers)
A polysaccharide formed up of two monosaccharides (in this case glucose). The polysaccharide in this case is a maltose molecule and is a disaccharide (di for 2 monomers)
Proteins (made only of Carbon, Hydrogen, Oxygen, Nitrogen, and Sulfur):
Proteins are polymers made of a chain of monomers called amino acids. There are 20 major amino acids that your body uses. You don't need to memorize the different amino acids, but you do need to know their general structure. All amino acids are composed of a central carbon bonded to an amino group, carboxyl group, single hydrogen, and R side chain.
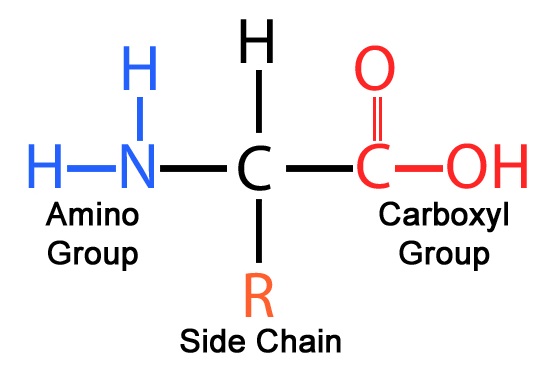 The general structure of a amino acid
The general structure of a amino acid
The difference between amino acids is always in their side chain. Side chains can have polar or nonpolar, and hydrophobic or hydrophilic properties. Once again, you do not need to know all of these side chains. The bond that connects two amino acids is called a peptide bond.
 The formation of a peptide bond via dehydration synthesis (described later in this article)
The formation of a peptide bond via dehydration synthesis (described later in this article)
Nucleic acids (made only of Carbon, Hydrogen, Oxygen, Nitrogen, and Phosphorous):
Nucleic acids are the most complex of the four major macromolecules because they are designed to store your body's genetic information. There are two general types of nucleic acids, DNA and RNA, which are differentiated by their sugar backbone (don't worry, this will be explained in a later lesson). Both nucleic acids are composed of monomers called nucleotides, which each store one piece of information (like a “bit” in programming).
 A diagram of a strand of DNA
A diagram of a strand of DNA
Lipids (made only of Carbon, Hydrogen, Oxygen):
Lipids are a slight exception to the rule. They are one of the major macromolecules, but they are not composed of monomers. They are always large nonpolar molecules. There are several types of lipids.
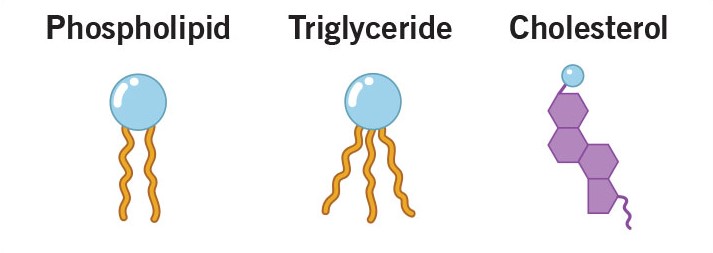 Three major types of lipids
Three major types of lipids
Dehydration synthesis and hydrolysis:
Dehydration synthesis is the process of joining (synthesizing) two monomers together. It involves the removal of a hydrogen atom (H) from one molecule and a hydroxyl group from another (OH), altogether removing H2O. It is called dehydration synthesis because you are dehydrating (removing a water) from the two molecules to join them.
Hydrolysis is the exact opposite of dehydration synthesis. It involves the addition of a water molecule to split two monomers.
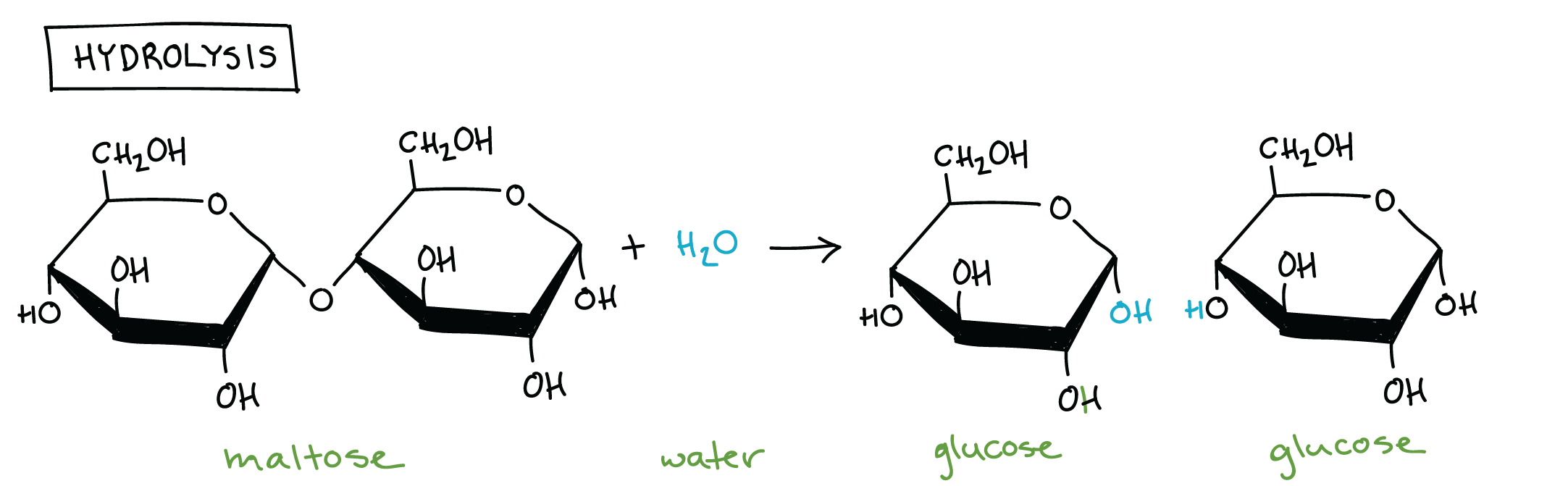 Hydrolysis of a maltose molecule into two glucose molecules
Hydrolysis of a maltose molecule into two glucose molecules