1.4: Properties of Biological Macromolecules
All important biological molecules in your body are macromolecules. These include carbohydrates, proteins, nucleic acids, and lipids. Topic 1.4 is about what makes up these macromolecules and their different properties.
Vocab List
- Properties of Carbohydrates
- Structural isomers
- Enantiomers
- α and β carbs
- Properties of Amino Acids
- R side chains
- Properties of Nucleic Acids
- Ribose
- Deoxyribose
- Nitrogenous base
- Adenine
- Thymine
- Guanine
- Cytosine
- Uracil
- Properties of Lipids
- Triglycerides
- Fatty acid chain
- Saturated fats
- Unsaturated fats
- Phospholipids
- Steroids
- Triglycerides
Written Explanation
Carbohydrates:
As explained in 1.0, an isomer is a molecule with the same molecular formula but a different molecular structure as another molecule. Carbohydrates have many isomers, including both structural isomers and enantiomers. Structural isomers have a different molecular arrangement, while enantiomers are just mirror images.
 Carbohydrate enantiomers
Carbohydrate enantiomers
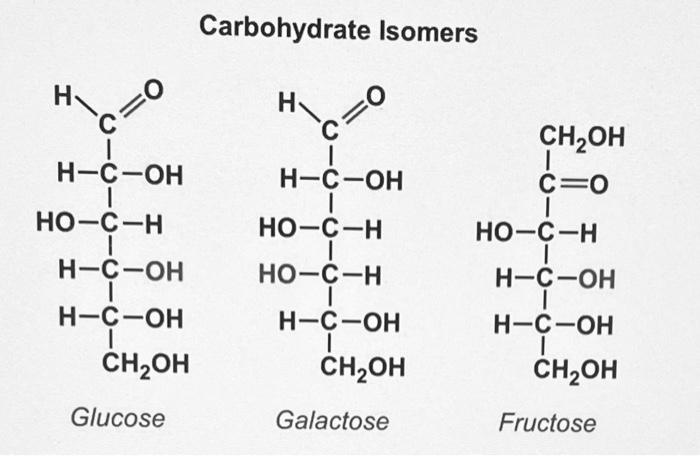 Carbohydrate structural isomers
Carbohydrate structural isomers
Different carbohydrate monomers are often linked together with bonds in different locations. Another way carbohydrates differ from each other is in their ring structure. They come in two variations (α and β). You don't need to know the specifics for these differences, but you do need to remember where they exist and how they affect the function of a molecule.
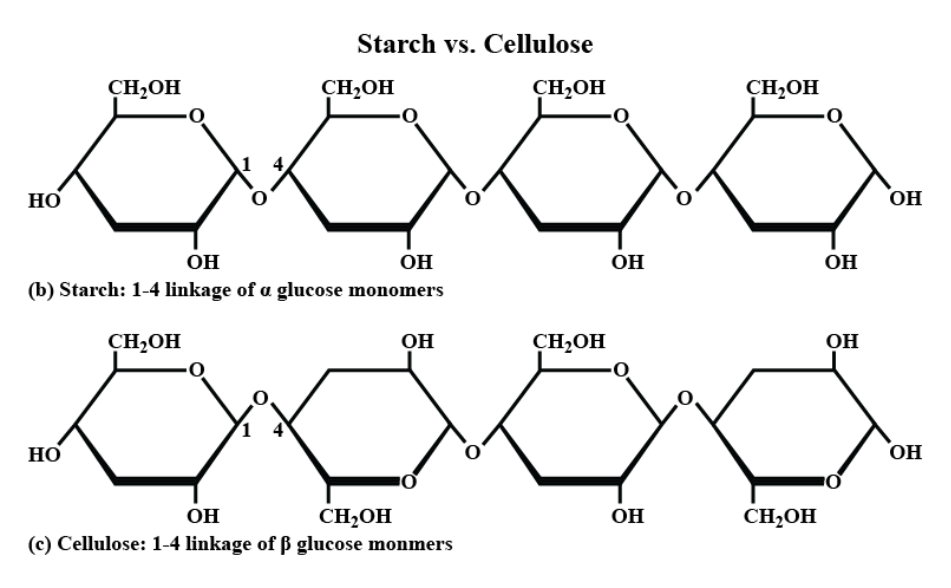 Diagram of starch compared to cellulose. It shows the difference between α and β glucose bonding
Diagram of starch compared to cellulose. It shows the difference between α and β glucose bonding
Amino Acids/Proteins:
All amino acids are composed of a main body and a R side chain. These side chains all have different properties, which contribute to the protein's final structure and function. The most important property to know is polarity.
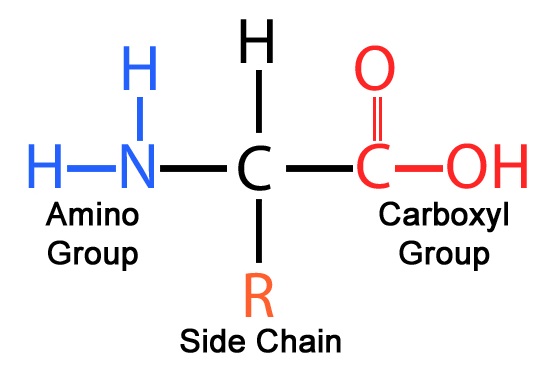 The general structure of a amino acid
The general structure of a amino acid
 The 21 main amino acids found in eukaryotes
The 21 main amino acids found in eukaryotes
Nucleic Acids:
Nucleic acids are polymers made of many nucleotides. All nucleotides consist of a 5 carbon sugar, a phosphate group, and a nitrogenous base.
:max_bytes(150000):strip_icc()/what-are-the-parts-of-nucleotide-606385-FINAL-5b76fa94c9e77c0025543061.png) The three parts of a nucleotide
The three parts of a nucleotide
In DNA (Deoxyribonucleic Acid), the 5 carbon sugars are deoxyribose (it's in the name), and the possible nucleic acids are ATCG (Adenine, Thymine, Cytosine, and Guanine). In RNA (Ribonucleic Acid), the 5 carbon sugars are ribose (once again, it's in the name), and the possible nucleic acids are AUCG (Thymine is swapped with Uracil). The details here are important, and you'll learn more about them later in this unit.
Lipids:
All lipids are hydrophobic (they "don't like" water). They come in three main variations: fats (triglycerides), steroids, and phospholipids.
Fat molecules all contain a glycerol molecule and 3 fatty acid chains (which are hydrocarbons). The fats come in two variations of their own, which you have likely already heard a lot about: saturated fats and unsaturated fats. These terms refer to how many double bonds the hydrocarbons have. Saturated fats have none, and unsaturated fats have some.
 A saturated fat molecule
A saturated fat molecule
 An unsaturated fatty acid chain
An unsaturated fatty acid chain
Steroids are 4 carbon rings. They help transport signals throughout the body. Hormones are a type of steroid.
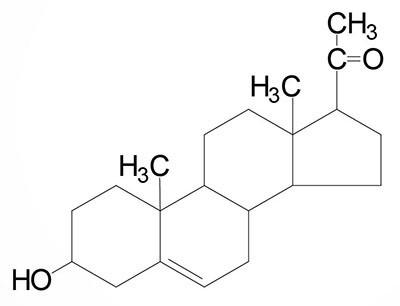 Generic steroid example
Generic steroid example
The last type of lipid is a phospholipid. These contain both a hydrophobic and a hydrophilic region. They are a major component of cell membranes and they form themselves into a bilayer (called a phospholipid bilayer).