1.5: Structure and Function of Biological Macromolecules
All important biological molecules in your body are macromolecules. These include carbohydrates, proteins, nucleic acids, and lipids. Topic 1.5 is about what these macromolecules do.
Vocab List
- Carbohydrates
- Starch
- Glycogen
- Cellulose
- Chitin
- Microfibrils
- Proteins
- Gene expression
- Primary structure
- Secondary structure
- α helix
- β pleated sheet
- Tertiary structure
- Disulfide bridges
- Hydrophobic interactions (van der Waals forces)
- Quaternary structure
- Denturation
- Nucleic Acids
- Antiparallel
- Nitrogenous bases
- Adenine
- Thymine
- Guanine
- Cytosine
- Uracil
- Purines
- Pyrmidines
- Lipids
- Cholesterol
Written Explanation
Carbohydrates:
Carbohydrates store short term energy and support the structure of cells and organisms as a whole. Energy is stored in the chemical bonds of the carbohydrates, with some carbs being able to store more energy more efficiently than others. Two carbs that are optimized for energy storage are starch (in plants) and glycogen (in animals). When broken down, these molecules release energy that helps run a cell. Both carbs are long glucose chains that differ slightly in how the glucoses are bonded. Due to their chained and branched structure, bonds between glucose molecules are more easily broken apart to release energy in starch and cellulose, making them good short term energy storing and providing molecules.
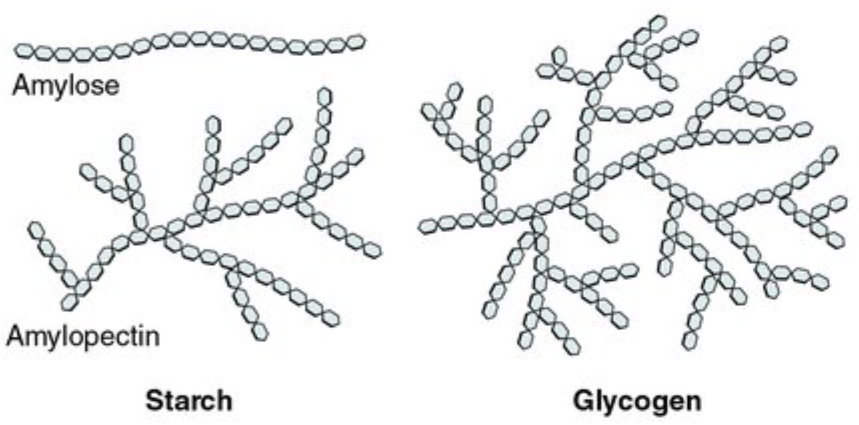 Diagram of starch and cellulose molecules. They are quite similar in structure, but just differ in how they are bonded together. The hexagons represent glucose monosaccharides
Diagram of starch and cellulose molecules. They are quite similar in structure, but just differ in how they are bonded together. The hexagons represent glucose monosaccharides
Cellulose is another glucose polymer, which forms the cell wall of plants. It is essentially a wall of glucose molecules, giving it the rigid and shapely structure needed to perform its function as a wall.
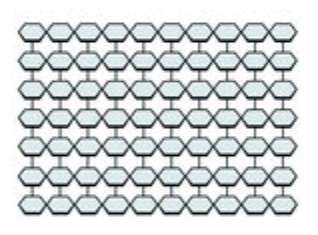 Diagram of a cellulose fiber. The hexagons represent glucose monosaccharides
Diagram of a cellulose fiber. The hexagons represent glucose monosaccharides
Chitin is another structural carbohydrate which forms the exoskeleton of lobsters, insects, and fungi. Lastly, cellulose microfibrils are structures in plant cell walls arranged in an organized crystalline structure.
Proteins:
Proteins have the most diverse functions of all the macromolecules. They act as catalysts in chemical reactions, store amino acids, move (muscles), defend the body (antibodies), play a part in cell transport (including receptors), support the structure of a cell, and build and repair parts of the body. Overall though, proteins exist to express genes. They carry out the instructions written in your DNA (nucleic acids).
Proteins also have a complex structure that is broken down into 4 major levels. The first level of structure is called a protein's primary structure. Primary structure is the sequence of amino acids that make up a protein (remember, proteins are a polymer made up of many amino acid monomers).
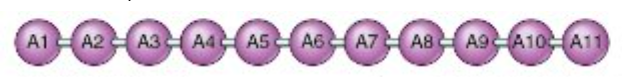 The primary structure of a protein shown as a chain of different amino acids
The primary structure of a protein shown as a chain of different amino acids
The secondary structure of a protein is how the backbones of amino acids are bonded. Secondary structure includes α helices and β pleated sheets. This level of structure is formed by hydrogen bonding between the backbones of individual amino acids, and provides a base structure for the protein.
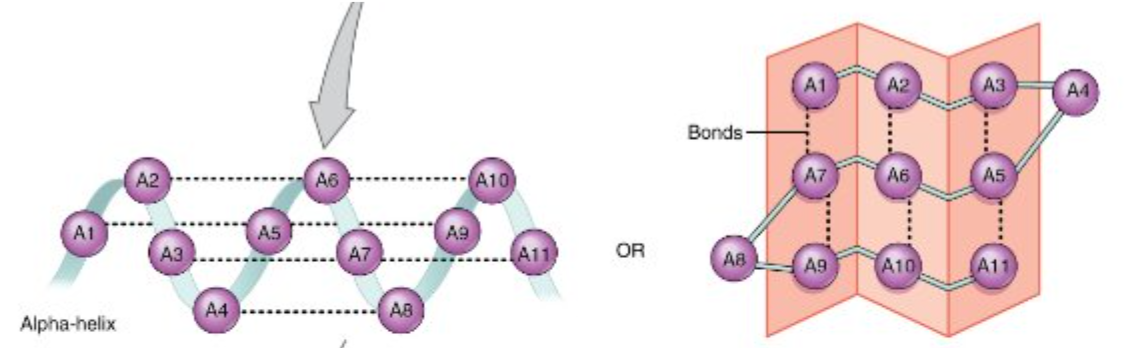 Diagram of secondary structure. An α helix is shown on the left, and a β pleated sheet is shown on the right.
Diagram of secondary structure. An α helix is shown on the left, and a β pleated sheet is shown on the right.
The tertiary structure of a protein is how the protein folds. This structure is formed by the interactions between the amino acids's R side chains (discussed in 1.3). In general, hydrophilic side chain amino acids fold outwards to the environment, while hydrophobic side chain amino acids curl inwards, leading to interactions between these side chains. The interactions include hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions such as van der Waals forces (see 1.0). Disulfide bridges are covalent bonds between two sulfhydryl groups and hydrophobic interactions are very weak bonds between hydrophobic side chains, formed from their close proximity to each other.
 Diagram of the different bonds and interactions within the tertiary structure of a protein
Diagram of the different bonds and interactions within the tertiary structure of a protein
The quaternary structure of a protein is how different chains of amino acids (polypeptides) interact with each other. Quaternary structure is not present in all proteins (specifically those which are composed of only one polypeptide), but is present in other proteins like hemoglobin, which consists of four different polypeptides. The bonds connecting the chains of amino acids in quaternary structure are the same as tertiary structure.
 A diagram of hemoglobin, with the four polypeptides that it is made up of shown in different colors
A diagram of hemoglobin, with the four polypeptides that it is made up of shown in different colors
When a protein's environment changes outside of its normal conditions, the protein may denature, meaning that it loses its shape. This can happen if the pH or temperature of the environment changes, causing the bonds of quaternary, tertiary, and secondary structure to break. Primary structure of the amino acid chain remains due to the strong covalent bonding between individual amino acids.
Nucleic Acids:
As previously mentioned, DNA is composed of two strands built out of nucleotides. The nucleotides are joined together in one strand through covalent bonds, but the two strands themselves bind together through hydrogen bonds between the nitrogenous bases of the nucleotides. These include adenine (A), guanine (G), cytosine (C), and thymine (T), and they can only bond together A-T and C-G. A good way to remember this pairing rule is through the phrase “Apple tree, car garage,” representing the first letter of each nitrogenous base. Additionally, A and G are made up of two carbon rings and are known as purines, while C and T are one ringed pyrimidines.
The two strands of DNA also run antiparallel, meaning that they are parallel but run in opposite directions. Both strands run from the 5' carbon to the 3' carbon, known as their directionality, but they are placed running in opposite directions. The numbering of the carbon is determined by counting from the oxygen atom in the deoxyribose of DNA nucleotides.
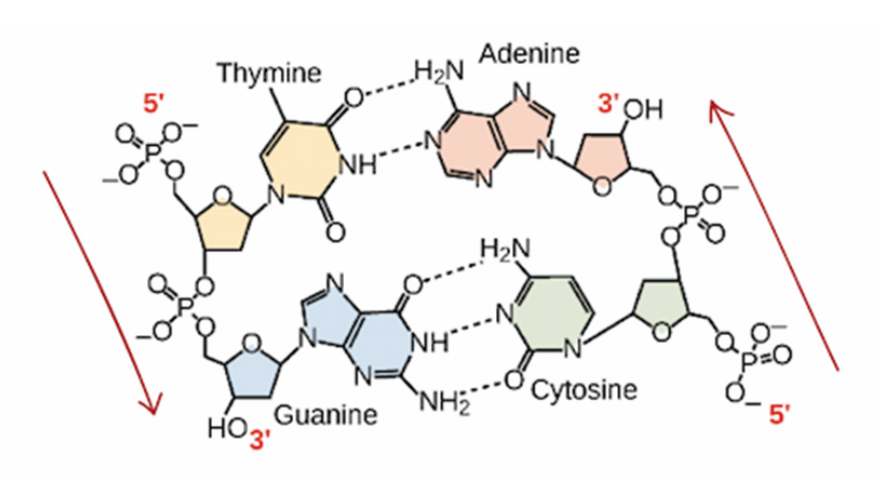 Two DNA strands running antiparallel, also showing the base pairing rule between nitrogenous bases
Two DNA strands running antiparallel, also showing the base pairing rule between nitrogenous bases
The role of nucleic acids is to store and express genetic information. DNA is not the only nucleic acid, and differences in the structures between different nucleic acids and how they perform this function will be discussed in topic 1.6.
Lipids:
Lipids function as long term energy storage, along with insulation and protection for vital organs. They are the main component of cell membranes in the form of phospholipids, and are also the macromolecules which make up hormones. The base lipid which all hormones are derived from is cholesterol.
 A cholesterol molecule
A cholesterol molecule
Lipids are used as sources of long term energy and not short term energy due to their structure. Due to the long hydrocarbon chains of triglycerides a lot of energy is stored between the hydrogen-carbon bonds but they are difficult to break, and are used only once short term energy from carbohydrates has run out.
 A triglyceride with 3 long hydrocarbon chains (fatty acids)
A triglyceride with 3 long hydrocarbon chains (fatty acids)