1.1: Structure of Water and Hydrogen Bonding
This lesson has to do with an essential component of life: water. Topic 1.1 covers the structure of water and the properties which make it such an important and life sustaining molecule. Although this topic isn't mentioned often by College Board it covers many essential concepts for the rest of the course. It is highly recommended that you go over chemistry fundamentals in topic 1.0 before starting this lesson.
Vocab List
- Electronegativity
- Polarity
- Hydrogen bonds
- Cohesion
- Surface tension
- Adhesion
- Meniscus
- Capillary action
- Transpiration
- High specific heat
- High heat of vaporization
- Solution
- Solvent
- Solute
- Hydrophilic
- Hydrophobic
Written Explanation
Water is composed of three atoms: one oxygen and two hydrogen (H2O). The two hydrogen atoms are covalently bonded to the oxygen atom forming a V shape. (A covalent bond is when two atoms share an electron to fill their outer valence electron shells.)
 Lewis dot structure of a water molecule
Lewis dot structure of a water molecule
However, the electrons do not hover around the atoms equally. Because oxygen has more protons than hydrogen, the electrons move slightly closer to it, giving oxygen a slightly negative charge (electrons are negative). An atom like this is called electronegative, meaning it wants to hog electrons. Electronegativity generally increases as you move up and to the right on the periodic table, precisely where oxygen is located. The hydrogen atoms, as a result of having the electrons further from them most of the time have a slightly positive charge (electropositive). This results in a partially negative charge on the side of the water molecule which contains the oxygen atom and a partially positive charge on the side of the hydrogen atoms. This makes water a polar molecule. (Polar means there is a partial charge.)
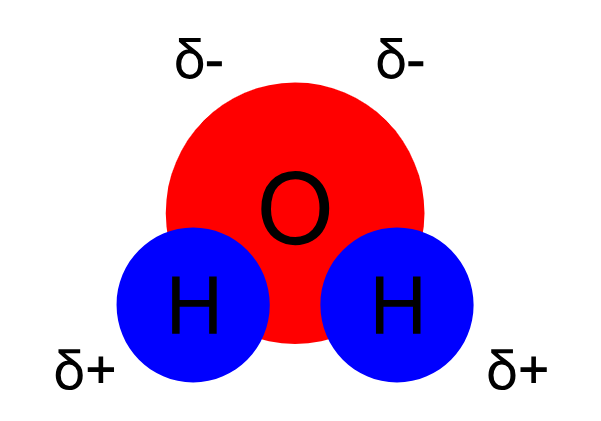 Diagram of water's polarity. The symbol δ means partial charge (δ+ is a partial positive and δ- is a partial negative charge)
Diagram of water's polarity. The symbol δ means partial charge (δ+ is a partial positive and δ- is a partial negative charge)
Hydrogen Bonds:
Just like with magnets and electricity, opposite charges attract, and therefore, these partial charges allow the positive and negative sides of different water molecules to form weak bonds with each other. These are known as hydrogen bonds, and they usually form between the oxygen atom of one molecule and the hydrogen atom of another. One water molecule can form up to 4 hydrogen bonds. (one per each hydrogen and 2 for each oxygen atom.) These bonds are extremely weak, and this is the key to some of water's other properties. Note: hydrogen bonds can also form between hydrogen and fluorine or hydrogen and nitrogen.
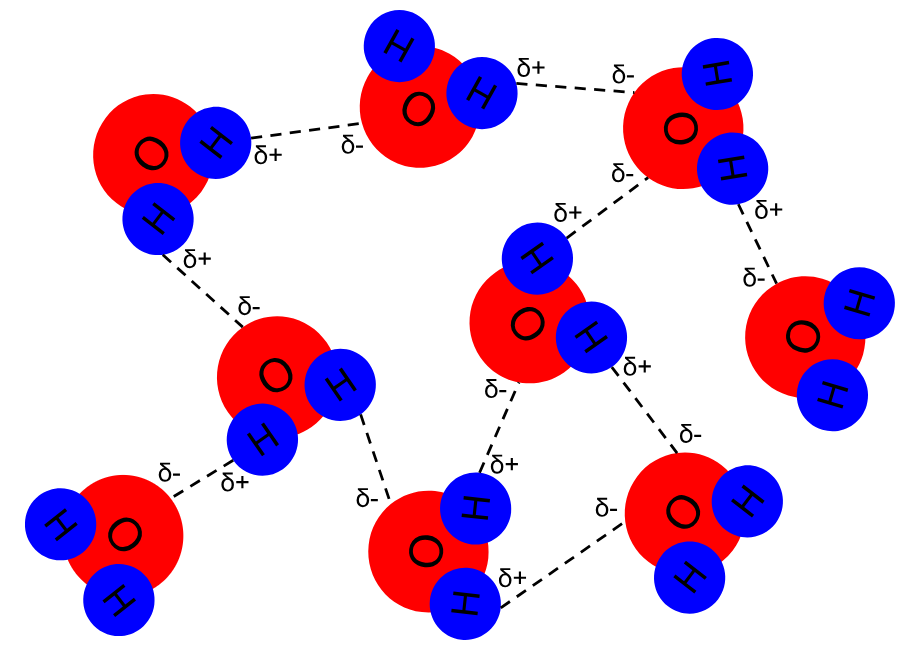 Diagram of hydrogen bonding in water
Diagram of hydrogen bonding in water
Cohesion and Adhesion:
All of the hydrogen bonds formed by water allow it to attract to itself or other polar objects. When water attracts itself, the property is called cohesion. This allows it to stay together, and partially resist gravity.
 Cohesion creates water droplets that stick together and don't spread out due to gravity
Cohesion creates water droplets that stick together and don't spread out due to gravity
Cohesion also allows the tops of bodies of water to stick together, a concept known as surface tension. Surface tension provides a surface for some small bugs to walk on. This property can be seen when slightly overfilling a cup without it spilling.
:max_bytes(150000):strip_icc():format(webp)/Surface-Tension-58c6c2365f9b58af5c534f71.jpg) Surface tension allows this bug to walk by creating a weak surface that resists the bug's weight
Surface tension allows this bug to walk by creating a weak surface that resists the bug's weight
When water sticks to polar molecules other than itself, that property is called adhesion. Water can only bind to other polar/charged substances. You might see this when filling water into a glass beaker. The water will creep slightly up the sides of the beaker, as the adhesion of the water to the glass is stronger than the pull of gravity and cohesion. The curved surface of the water in this case is called a meniscus. Adhesion also allows water to climb up a paper towel, an event known as capillary action.
 Capillary action and adhesion in a beaker and tube
Capillary action and adhesion in a beaker and tube
Lastly, water uses its adhesive and cohesive properties together to climb the stems of plants during transpiration, which is just a fancy word for evaporation from plants. Water is able to stick to the xylem tube of the stem through adhesion and pull itself up against gravity using capillary action, a process started by the power of the sun. Remember, these properties are only possible due to hydrogen bonding, which in turn are only possible due to water's polarity!
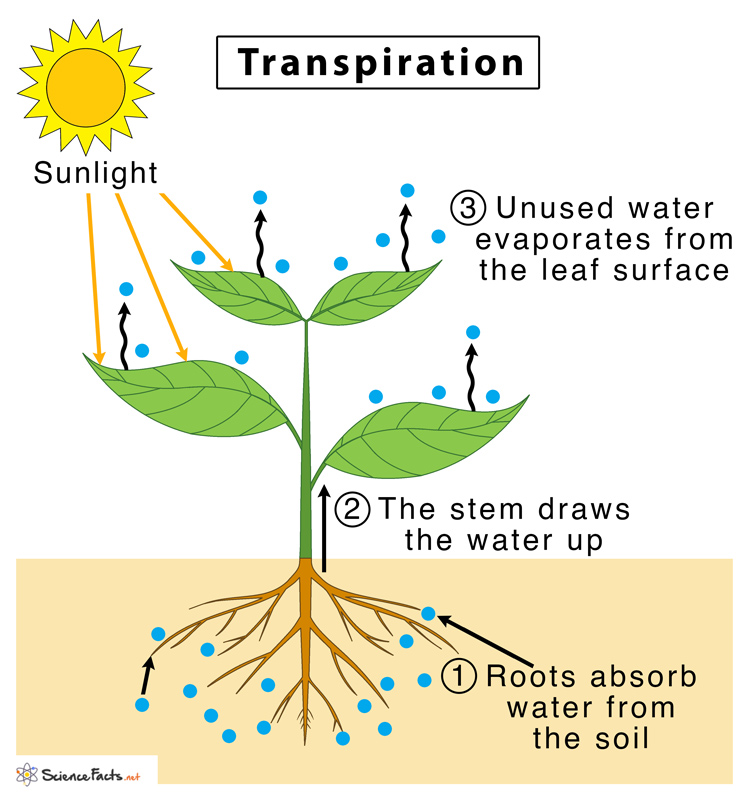 Diagram of transpiration in a plant
Diagram of transpiration in a plant
Specific Heat:
Water also has a very high specific heat (specifically 4.186 J/g°C), meaning that raising 1g of water by 1 degree Celsius requires more energy than raising 1g of sand by 1°C. This means water takes both longer to warm up and cool down, which allows it to regulate temperature (the same amount of energy is used over time, but that energy takes longer to have an effect). This property of water helps creatures living in bodies of water and also acts as the reason for coastal cities' more moderate climates. The reason for this high specific heat lies in the hydrogen bonds between water molecules. Before the temperature of the water can be raised, the hydrogen bonds between water molecules have to be broken. Before the temperature can be lowered, the bonds must be built (the bonds require/store energy). This allows water to absorb a lot of heat before the bonds actually break and the temperature is raised. Moreover, this energy absorption also takes part in evaporative cooling. When you sweat, the water on your skin absorbs energy (heat) from your skin, and when it evaporates that energy is taken away.
Density:
Next, water has a lower density as a solid than liquid. Thus, when you place ice into water, it will float. Just think of a cool drink on a summer day. This is interesting because in most substances the solid form is more dense than the liquid, meaning they will sink instead of float. This property of water is caused by water molecules (still connected by hydrogen bonds) spacing out to form a crystalline structure as they cool down and results in the safety of animals living in ponds and lakes, such as when the body of water freezes over in the winter the ice is situated at the top, allowing life to continue below it.
Notice how water molecules are more evenly distributed in solid form than in liquid form, leading to a higher density in ice.
Solvent properties:
Lastly, water is considered to be an almost universal solvent. A solvent is a substance that dissolves another substance (the solute) to form a solution. Water can dissolve almost any polar or ionic molecule. Substances such as these are called hydrophilic, or water loving. Those which dislike water and are non-polar are hydrophobic. A common example of water's solvent properties is putting table salt into water and watching it dissolve. Table salt's chemical formula is NaCl, and it is formed through an ionic bond between the sodium ion (Na+) and the chlorine ion (Cl-). When placed into water, the positive sodium ion gets bonded to the partially negatively charged oxygen atom of the water molecules, while the negative chlorine ion becomes attracted to the partially positively charged hydrogen atoms within water. As the water molecules arrange themselves around the two ions, the difference in how they attach to the ions result in a separation of the two ions and a cleavage of the ionic bond within salt, dissolving it. Water's power as a solvent also allows it to take nutrients with it as it travels through the water cycle.